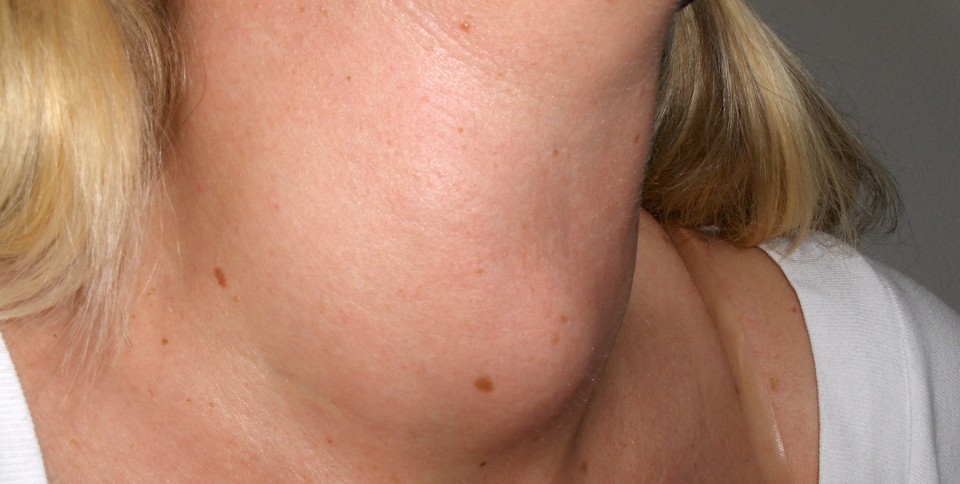
Hyperthyroidism or thyroxicosis is a condition that encompasses several different diseases, all of which are characterized by hypermetabolism and elevated serum levels of free thyroid hormones. It is the result of the excess secretion of thyroid hormones from the thyroid gland, with or without their increased synthesis. The most common causes of hyperthyroidism include Grave’s disease, toxic multinodular goiter and toxic adenoma.
Graves disease
Graves’ disease is the most common cause of hyperthyroidism, and is an autoimmune disease that has a chronic course with remissions and relapses. The etiology of Graves’ disease relates to the production of IgG antibodies against the thyroid TSH receptor, which results in the continuous stimulation of the gland to synthesize and secrete excess quantities of T4 and T3. It is sometimes associated with other autoimmune disorders, including insulin-dependent diabetes mellitus, vitiligo, premature graying of hair, pernicious anemia, collagen diseases, and polyglandular deficiency syndrome. It is far more common in women than in men, and has been observed to occur in periods of emotional stress. (Berkow 1992, 1075-77; Rubin 2001, 604-05)
The clinical features of Grave’s disease typically involve a gradual onset of non-specific symptoms such as nervousness, emotional irritation, tremor, weakness, weight loss and tachycardia. Patients are intolerant of heat and tend to sweat profusely. One of the more striking features of Grave’s is exophthalamos, a protruding of the eyeballs characterized by the enlargement of intraocular muscles from the accumulation of fluid, fibroblasts and lymphocytes. The thyroid is often symmetrically enlarged from increased metabolic activity. (Berkow 1992, 1075-77; Rubin 2001, 604-05)
The thyroid storm of hyperthyroidism is characterized by the rapid onset of acute symptoms of hyperthyroidism, including fever, marked weakness and muscle wasting, extreme restlessness with emotional irritability, confusion, psychosis, hepatomegaly with mild jaundice. The patient may present with cardiovascular collapse and shock, and is a life-threatening emergency. (Berkow 1992, 1076)
Toxic nodular goiter
Toxic nodular goiter (TNG) is the second most common cause of hyperthyroidism after Graves disease in the Western world, representing about 15-30% of cases, and in areas of endemic iodine deficiency it is the most common cause. TNG represents a spectrum ranging from a single hyperfunctioning nodule within a thyroid that contains other nonfunctioning nodules to multiple areas of hyperfunctioning nodules throughout the gland. TNG is most often seen to arise from a simply goiter, secondary to an iodine deficiency. A deficiency of iodine results in low levels of circulating thyroxine (T4), and through a feedback mechanism, promotes thyroid cell hyperplasia. Increased thyroid cell replication predisposes single cells in the thyroid to mutations of the TSH receptor, resulting in the formation of multiple nodules. (Berkow 1992, 1077; Rubin 2001, 605-06)
Toxic adenoma
Toxic adenoma (Plummer’s disease) refers to a solitary, hyperfunctioning follicular neoplasm in an otherwise normal thyroid. These neoplasms function independent of TSH, and the hyperactiovity of the nodule eventually suppresses the remainder of the thyroid, which then atrophies. As the condition progresses TSH levels decline. It is most common in the fourth and fifth decades of life, but the patients do not typically express symptoms of hyperthyroidism until the adenoma has grown more than 3 cm in diameter. Adenomas are typically treated by surgery. (Berkow 1992, 1077; Rubin 2001, 605-06)
Drug-induced thyroid dysfunction
There are several drugs that are known to cause thyroid dysfunction. Among these are the antihypertensive drug aminodarone, taken concurrently with a low intake of iodine, more common in the elderly (Trivalle et al 1996). Another cause is lithium therapy, which appears to impair the process of deiodination of T4 peripherally and within cells, inhibiting its conversion to T3 (Oakley 2002). Lithium is commonly used in psychiatry in the treatment of manic episodes of manic-depression. While other psychiatric drugs have not been associated with thyroid dysfunction, the author has observed the spontaneous manifestation of thyrotoxicosis with other psychiatric drugs such as risperidone. While the effects of the long term use of these drugs upon thyroid function has not been properly studied, these drugs are associated with an acute, potentially condition called neuroleptic malignant syndrome (NMS), manifesting as typical hyperthyroid-like symptoms including hyperpyrexia, muscle rigidity, altered mental status, tachycardia, diaphoresis, and cardiac dysrhythmia.
Diagnosis of hyperthyroidism
The diagnosis of hyperthyroidism is concluded by noting the elevation of T3 and T4, and/or by the increased uptake of radioactive iodine. TSH levels are typically very low or undetectable. In Graves’ disease specifically, the antibody against the thyroid TSH receptor is measured, usually with an enzyme-linked immunosorbent assay (ELISA) for anti-TPO antibody (thyroperoxidase). (Berkow 1992, 1075-77; Rubin 2001, 604-05)
If the etiology remains unclear the patient will typically undergo a nuclear thyroid scintigraphy iodine 123 (I-123) uptake and scan test. Graves disease is characterized by a diffuse enlargement of both thyroid lobes, with an elevated uptake of I-123. TNG shows up as an enlarged thyroid with multiple nodules and areas of both increased and decreased uptake. A toxic adenoma is characterized a by a solitary “hot nodule” that takes up the isotope in an otherwise non-functional thyroid. If the nodule is “cold” (i.e. does not take up the isotope), a biopsy of the nodule is performed by fine-needle aspiration to exclude cancer. (Berkow 1992, 1075-77; Rubin 2001, 604-05)
Medical treatment
The primary focus of treatment of hyperthyroidism consists of symptomatic relief, treatment with antithyroid medications, the use of radioactive iodine 131 (I-131), or thyroidectomy. The neurological and cardiovascular symptoms of thyrotoxicosis are treated by beta-blocker or calcium channel blocker, concurrent with oral rehydration therapy. Specific antithyroid medications include methimazole and propylthiouracil, which inhibit the synthesis of T4 and T3 by inhibiting the formation and coupling of iodotyrosines in thyroglobulin, leading to a gradual reduction in thyroid hormone.Although methimazole is a longer-acting drug, propylthiouracil dosed more frequently in severe thyrotoxicosis due to a specific inhibition in the conversion of T4 into T 3. Adverse effects include allergic drug reactions such as fever, rash, urticaria, and arthralgia, as well as more serious adverse effects such as agranulocytosis, aplastic anemia, hepatitis, polyarthritis, and a lupus-like vasculitis. (Berkow 1992, 1075-77)
Radioactive iodine-131 therapy is the most common treatment of hyperthyroidism, administered orally as a single dose, in capsule or liquid form. The radioactive iodine is quickly absorbed and taken up by the thyroid. The treatment results in a thyroid-specific inflammatory response, causing fibrosis and destruction of the thyroid over weeks to many months. This treatment is typically avoided in younger patients and is contraindicated in pregnancy. About 60% of patients become hypothyroid after treatment (Aizawa et al 1997).
Surgical options for hyperthyroidism consist of a subtotal thyroidectomy or a total thyroidectomy, or combinations of hemithyroidectomies and contralateral subtotal thyroidectomies. Thyroidectomy is typically reserved for severe hyperthyroidism in children and pregnant women.(Berkow 1992, 1077)
Holistic treatment
Hyperthyroidism is a typical heat-related disorder, and while no specific pathology is described in Ayurvedic medicine that correlates to our modern understanding of the disease, the signs and symptoms are clearly related to a severe pitta disorder. The underlying etiology of hyperthyroidism from an Ayurvedic perspective relates to the accumulation of ama, which aggravates pitta, and with the resultant heat pitta consumes ojas, leading to progressive weakness and debility. Thus treatment is orientated towards alleviating the specific manifestations, which may include similar diseases including jvara (‘fever’), daha (‘burning sensations’), trishna (‘thirst’), and atisara (‘diarrhea’). In Chinese medicine hyperthyroidism is related a combination of Liver fire rising, deficiencies of qi and yin, and Phlegm stagnation. The excess production of thyroid hormones correlates to Liver fire rising, which gradually promotes deficiencies in qi and yin. Phlegm stagnation specifically relates to the enlargement of the thyroid gland.
From a nutritional perspective, hyperthyroidism may be facilitated by both deficiencies and excess amounts of certain nutrients and toxic compounds found in the modern diet and environment. Among the most common nutrient deficiencies found in hyperthyroidism are vitamins A, B, C, and E, as well as copper, iron and sulfur. There is a good deal of evidence that copper is an essential nutrient to consider in hyperthyroid states, with animal studies showing increases in serum T3 levels in concurrent copper deficiencies (Kralik et al 1996). Vitamin A and the B vitamins (PABA, pantothenic acid, thiamine,riboflavin, and niacin) appear to be important in regulating copper metabolism. Zinc acts as a copper antagonist and high doses of zinc without concurrent copper supplementation may predispose or worsen hyperthyroid states (in normalcy the zinc:copper ratio should be 5:1 in women, and 10:1 in men). In several studies hyperthyroidism is correlated with a relative deficiency of RBC, which by some interpretations, is reason to supplement for zinc. One study however showed that with the use of antithyroid drugs RBC zinc normalized without supplementation (Yoshida et al 1990). In another study, patients with hyperthyroidism had a lower RBC zinc level and increased urinary zinc excretion. Body zinc levels, excluding plasma zinc, was positively correlated with plasma thyroid hormone levels (Tsou et al 1993). This suggests that hyperthyroid patients may be trying to excrete zinc as an adaptive mechanism to inhibit thyroid stimulation, and that when thyroid levels are normalized, bioavailable RBC zinc levels also normalize. Given that the ratios for zinc to copper are lower for women than in men, it would suggest that an increased requirement for copper would predispose women to suffer the negative effects of a relative copper deficiency.
Other compounds that appear to play a role in promoting hyperthyroidism include cadmium (from environmental contaminants and smoking), mercury (from amalgams and environmental contamination), lithium, and aluminum. As women are more susceptible to hyperthyroid states, the role of estrogen has recently been questioned. Specifically, it appears that estrogens may act as an accelerator of mineral uptake into the body, and in the presence of nutrient deficiencies could promote mineral imbalances in the body. Estrogen may act to enhance cadmium absorption, which accelerates thyroid function, and in a relative zinc excess, promote a copper deficiency. Similarly, if zinc levels are too low, estrogen can have the opposite effect, causing the body to incorporate too much copper and not enough zinc and thereby promoting hypothyroidism. Environmental and dietary estrogens appear to act synergistically with endogenous estradiol to promote heavy metal toxicity. In particular, xenoestrogens such as bisphenol-A (found in lacquers used to coat the inside of cans, and in dental sealants used to protect teeth) may act as potent xenoestrogenic compounds, which may explain why cats fed canned cat food have up to 11 times the risk of hyperthyroid diseases than cats not fed canned food.
Another important consideration on the mineral status of hyperthyroid patients is the concurrent manifestation of intestinal damage, mediated through gluten intolerances (i.e. celiac disease), inflammatory bowel disease, or the consumption of phytate-containing foods that interfere with mineral absorption. Many of these foods, such as grains, cereal and legumes, as well as dairy and other foods that alter the gut flora (e.g. sugar, flour) could also directly promote damage to the gut wall, and promote immunological cross-tolerances and antibodies directed against the TSH receptor, as is seen in Grave’s disease.
As hyperthyroidism is a life-threatening condition all treatments must be closely monitored by a licensed physician.
1. Ensure proper nutrition.
- oral rehydration
- Goitrogens (e.g. legumes, cruciferous vegetables) are theoretically used to inhibit thyroid production, but have been shown to be of little practical value, and in TNG, could exacerbate the underlying condition.
- Given that Grave’s disease is an autoimmune disorder, dietary strategies should be implemented to inhibit autoimmunity (see the Paleolithic diet)
- Iodine intake is usually restricted in hyperthyroidism, but a deficiency is an underlying mechanism in TNG. Small amounts of organic iodine may be helpful in hyperthyroid states, best consumed as sea vegetables, 3-5 g daily
- Ensure adequate vitamin A (e.g. liver, egg yolks, butter), 10,000 IU/day
- Ensure adequate B vitamins (e.g. liver, egg yolks, leafy greens, yeast),100 mg/day
- Ensure adequate vitamin C (e.g. fruit, vegetables), 2-3 g daily
- Ensure adequate vitamin D
- Ensure adequate vitamin E (e.g. nuts, seeds, egg yolks, butter), 400-800 IU/day
- Ensure adequate omega-3 fatty acids, EPA/DHA, 1000 mg each daily
- Ensure adequate selenium (e.g. halibut, snapper, salmon, brazil nuts, clams, oysters), 200 mcg daily
- Ensure adequate copper (e.g. organ meat, eggs, nuts, seeds), 5 mg/day; this should be the first mineral supplemented, followed by iron and sulfur, and then zinc
- Ensure adequate iron (e.g. liver, meat, eggs, leafy greens), 10-20 mg daily
- Ensure adequate sulfur (e.g. eggs, cruciferous vegetables, alliums), and/or as MSM, 500 mg – 3 g daily
- Assess zinc status, carefully supplement after copper administration, 5-15 mg/day
2. Decrease thyroid function, reduce heat, and nurture the vital energy.
- Western botanicals to downregulate thyroid function, e.g. Bugleweed (Lycopus virginicus), Balm (Melissa officinalis), Motherwort (Leonorus cardiaca), Basil (Ocimum basilicum), Self Heal(Prunella vulgaris), Vervain (Verbena officinalis), Marshmallow (Althaea officinalis), Gromwell(Lithospermum officinalis)
- Indian botanicals to decrease pitta: Neem (Azadirachta indica), Chirata(Swerta chirata), Katuka (Picrorrhiza kurroa), Bhunimba(Andrographis paniculata), Guduchi(Tinospora cordifolia),Bhumyamalaki (Phyllanthus amarus), Sandalwood(Santalum album), Bhrngaraha (Eclipta alba), Kusmanda(Benincasa hispida) (unripe fruit), Nirgundi (Vitex negundo)flower, Nagakesara (Mesua ferrea) flower, Usira (Vetiveriazizanoides), Vamsa (Bambusa arundinacea) (manna),jaggery, ghee
- Chinese botanicals to reduce Liver fire: Gypsum, Huang Qin (Scutellaria baicalensis),Huang Lian (Coptidis chinensis), uncured Shi Di Huang (Rehmannia glutinosa) root, Jue Ming Zi (Cassia obtusifolia) seed, Mi Meng Hua (Buddleia officinalis) bud, Mu Dan Pi (Paeonia suffructicosa), Yellow Gentian (Gentiana lutea), Chuan Bei Mu (Fritillaria cirrhosa), Sha Shen (Adenophora tetraphylla), Huang Bai (Phellodendron amurense)
- Indian and Chinese botanicals to enhance ojas and supplement yin and qi: Shatavari (Asparagus racemosa), American Ginseng (Panax quinquefolium), Shi Hu (Dendrobium nobile), Gokshura(Tribulus terrestris), Licorice (Glycyrrhiza glabra), Huang Qi (Astragalus membranaceus), Shan Yao (Dioscorea opposita), Jubube date (Ziziphus jujuba), He Shou Wu (Polygonum multiflorum), Bai Shao (Paeonia alba), Gou Qi Zi (Lycium barbatum)
3.Modulate immune function.
- implement dietary changes to remove antigenic foods (e.g. dairy, cereals, grains, and legumes)
- modulate immune function using botanicals such as Grifola (Grifola umbellata),Reishi (Ganoderma lucidum), Ashvagandha (Withania somnifera), Licorice (Glycyrrhiza glabra), Ginseng (Panax spp), Turmeric (Curcuma longum), Shu Di Huang (Rehmannia glutinosa), Dan Shen (Salvia miltiorrhiza)root, Huang Qin (Scutellaria baicalensis), Chai Hu (Bupleurum chinense)
4.Topical therapies
- Sandalwood paste, prepared in milk, applied over fore head
- Sandalwood decocted in milk, sponged onto body
- Cold water baths